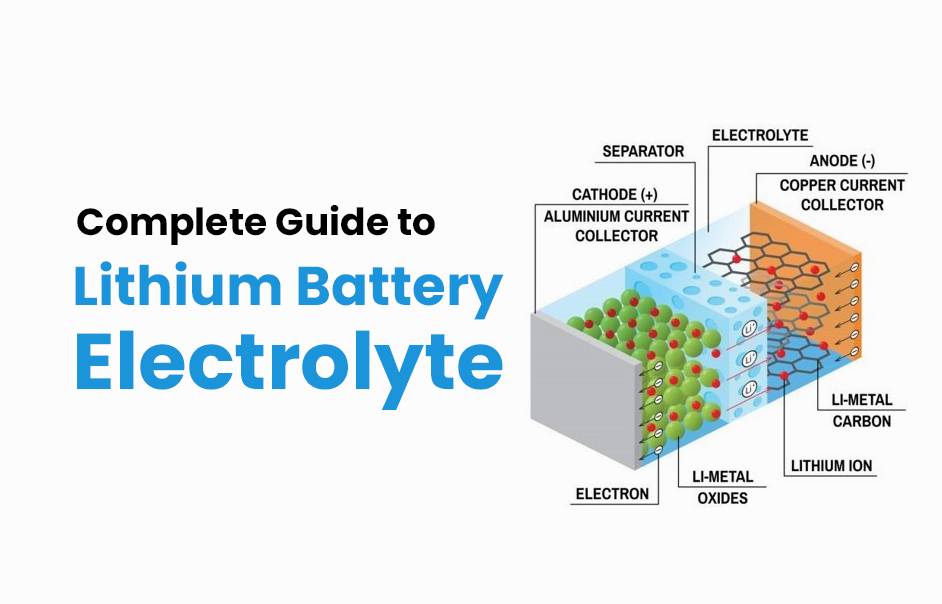
- Forklift Lithium Battery
-
48V
- 48V 210Ah
- 48V 300Ah
- 48V 420Ah (949 x 349 x 569 mm)
- 48V 420Ah (950 x 421 x 450 mm)
- 48V 456Ah
- 48V 460Ah (830 x 630 x 590 mm)
- 48V 460Ah (950 x 421 x 450 mm)
- 48V 460Ah (800 x 630 x 600 mm)
- 48V 460Ah (820 x 660 x 470 mm)
- 48V 500Ah
- 48V 560Ah (810 x 630 x 600 mm)
- 48V 560Ah (950 x 592 x 450 mm)
- 48V 600Ah
- 48V 630Ah
-
48V
- Lithium Golf Cart Battery
- 12V Lithium Battery
12V 150Ah Lithium RV Battery
Bluetooth App | BCI Group 31
LiFePO4 Lithium
Discharge Temperature -20°C ~ 65°C
Fast Charger 14.6V 50A
Solar MPPT Charging - 24V Lithium Battery
- 36V Lithium Battery
- 48V Lithium Battery
-
48V LiFePO4 Battery
- 48V 50Ah
- 48V 50Ah (for Golf Carts)
- 48V 60Ah (8D)
- 48V 100Ah (8D)
- 48V 100Ah
- 48V 100Ah (Discharge 100A for Golf Carts)
- 48V 100Ah (Discharge 150A for Golf Carts)
- 48V 100Ah (Discharge 200A for Golf Carts)
- 48V 150Ah (for Golf Carts)
- 48V 160Ah (Discharge 100A for Golf Carts)
- 48V 160Ah (Discharge 160A for Golf Carts)
-
48V LiFePO4 Battery
- 60V Lithium Battery
-
60V LiFePO4 Battery
- 60V 20Ah
- 60V 30Ah
- 60V 50Ah
- 60V 50Ah (Small Size / Side Terminal)
- 60V 100Ah (for Electric Motocycle, Electric Scooter, LSV, AGV)
- 60V 100Ah (for Forklift, AGV, Electric Scooter, Sweeper)
- 60V 150Ah (E-Motocycle / E-Scooter / E-Tricycle / Tour LSV)
- 60V 200Ah (for Forklift, AGV, Electric Scooter, Sweeper)
-
60V LiFePO4 Battery
- 72V~96V Lithium Battery
- Rack-mounted Lithium Battery
- E-Bike Battery
- All-in-One Home-ESS
- Wall-mount Battery ESS
-
Home-ESS Lithium Battery PowerWall
- 24V 100Ah 2.4kWh PW24100-S PowerWall
- 48V 50Ah 2.4kWh PW4850-S PowerWall
- 48V 50Ah 2.56kWh PW5150-S PowerWall
- 48V 100Ah 5.12kWh PW51100-F PowerWall (IP65)
- 48V 100Ah 5.12kWh PW51100-S PowerWall
- 48V 100Ah 5.12kWh PW51100-H PowerWall
- 48V 200Ah 10kWh PW51200-H PowerWall
- 48V 300Ah 15kWh PW51300-H PowerWall
PowerWall 51.2V 100Ah LiFePO4 Lithium Battery
Highly popular in Asia and Eastern Europe.
CE Certification | Home-ESS -
Home-ESS Lithium Battery PowerWall
- Portable Power Stations
What You Need to Know About Lithium Battery Explosions

Lithium-ion batteries can explode under certain conditions, primarily due to thermal runaway, overcharging, or physical damage. Understanding the causes and prevention methods is crucial for safely using these batteries in various applications, from consumer electronics to electric vehicles.
What Causes Lithium-Ion Batteries to Explode?
Lithium-ion batteries can explode due to several factors:
- Thermal Runaway: This occurs when the battery overheats, leading to a chain reaction that causes further temperature increases and potential explosions.
- Overcharging: Charging beyond the recommended voltage can damage the battery’s internal structure, leading to failure.
- Physical Damage: Punctures or dents can compromise the battery’s integrity, causing short circuits and fires.
Chart: Causes of Lithium-Ion Battery Explosions
| Cause | Description |
|---|---|
| Thermal Runaway | Chain reaction due to overheating |
| Overcharging | Exceeding voltage limits |
| Physical Damage | Punctures or dents leading to internal shorting |
How Can You Prevent Lithium Battery Explosions?
Preventing lithium battery explosions involves several best practices:
- Use Quality Chargers: Always use chargers designed for your specific battery type.
- Avoid Overcharging: Disconnect the charger once the battery is fully charged.
- Store Properly: Keep batteries in a cool, dry place away from direct sunlight and extreme temperatures.
Chart: Prevention Methods
| Method | Description |
|---|---|
| Use Quality Chargers | Ensures safe charging |
| Avoid Overcharging | Prevents damage from excessive voltage |
| Store Properly | Reduces risk from heat and humidity |
What Are the Common Signs of Battery Failure?
Recognizing signs of battery failure can help prevent dangerous situations:
- Swelling: A swollen battery indicates gas buildup and potential failure.
- Heat: Excessive heat during charging or use is a warning sign.
- Leakage: Any fluid leaking from the battery is a serious concern.
Chart: Signs of Potential Battery Failure
| Sign | Implication |
|---|---|
| Swelling | Indicates possible failure |
| Excessive Heat | Warning of overheating issues |
| Leakage | Serious risk; stop using immediately |
Why Is Proper Charging Important for Lithium Batteries?
Proper charging is vital because:
- Prevents Overheating: Using the correct charger helps maintain safe temperature levels during charging.
- Extends Lifespan: Proper charging practices can prolong the overall life of the battery.
- Enhances Safety: Reduces risks associated with overcharging and thermal runaway.
Chart: Importance of Proper Charging
| Importance | Description |
|---|---|
| Prevents Overheating | Maintains safe operating temperatures |
| Extends Lifespan | Reduces wear and tear on battery components |
| Enhances Safety | Minimizes risks of accidents |
How Should You Store Lithium Batteries Safely?
To store lithium batteries safely:
- Keep in a Cool Environment: Store batteries at room temperature, ideally between 20°C and 25°C (68°F – 77°F).
- Avoid Moisture: Keep batteries dry and away from humid environments.
- Use Fireproof Storage Containers: Consider using fireproof bags or containers for added safety.
Chart: Safe Storage Practices
| Practice | Description |
|---|---|
| Cool Environment | Helps prevent overheating |
| Avoid Moisture | Reduces risk of corrosion |
| Fireproof Containers | Provides additional safety measures |
Tips for Battery Wholesale Buyers: How to Choose a Reliable Manufacturer?
When considering wholesale purchases or OEM orders for batteries, it’s crucial to choose a reliable manufacturer. Here are some tips:
- Research Manufacturer Reputation: Look for established companies like Redway Power, known for quality and reliability.
- Evaluate Product Range: Ensure they offer various battery types suitable for your needs.
- Check Certifications: Confirm compliance with industry standards.
For OEM orders from a reputable manufacturer like Redway Power, which has over 13 years of experience in lithium battery manufacturing, ensure clear communication regarding specifications and delivery timelines. This approach helps secure high-quality products that serve as excellent alternatives to lead-acid batteries.
Redway Power Expert Views
“Understanding the risks associated with lithium-ion batteries is essential for safe usage. By following proper charging and storage practices, users can significantly reduce the likelihood of incidents,” states an expert from Redway Power.
What to Do in Case of a Lithium Battery Explosion and Fire?
In the unfortunate event of a lithium battery explosion, taking immediate action is crucial for minimizing damage and ensuring safety. Follow these steps:
- Evacuate the Area: Quickly move away from the explosion site to reduce exposure to harmful fumes or smoke.
- Call for Help: Dial emergency services immediately and provide details for guidance on handling the aftermath.
- Do Not Attempt to Extinguish Fires: Avoid touching or trying to put out fires resulting from the explosion, as they may involve hazardous materials.
- Seek Medical Attention: If there are injuries, seek medical assistance promptly, even for minor injuries that could have long-term consequences.
- Preserve Evidence: If possible, document the scene with photos or videos before leaving for later documentation.
Prioritize personal safety, rely on expert advice, and remember that each lithium battery explosion scenario is unique.
FAQs
What are the recommended tools and methods for lithium battery fires?
What are the main causes of lithium battery fires and explosions?
Why are lithium batteries flammable and explosive, and how to use them safely?
Safety Precautions for Handling Lithium Batteries
What Are the Downsides of Lithium Batteries?
Can Lithium Batteries Pose Health Risks?
What Is the Main Problem with Lithium Batteries?
What Affects the Safety of Lithium Polymer Batteries?
Are Lithium Polymer Batteries Safe?
More FAQs
What are some detailed causes of battery explosion, such as large internal polarization, reaction with electrolyte, poor sealing performance, and high external ambient temperature?
Certainly! Battery explosions can be caused by various factors such as large internal polarization, which can lead to swelling due to gas generation from reactions between the pole and the electrolyte. Poor quality and performance of the electrolyte itself can also contribute to battery explosion incidents. Insufficient liquid injection during the manufacturing process or poor sealing performance in the assembly process can result in air leaks, causing the battery to bulge. Additionally, the presence of dust or pole piece dust can create micro-short circuits, increasing the risk of explosion. Issues like thicker positive and negative electrode pieces or the shell wall as well as poor liquid injection sealing can also contribute to battery explosions. Furthermore, high external ambient temperatures can exacerbate the situation and serve as a significant factor in causing battery explosions.
Can overcharge, over-current, ultrasonic welding, spot welding, over-discharge, vibration, falls, and other factors contribute to lithium battery explosions?
Various factors such as overcharge, over-current, ultrasonic welding, spot welding, over-discharge, vibration, falls, and others can indeed contribute to lithium battery explosions. For instance, overcharge can lead to an explosion by causing the electrolyte to decompose and generating excess pressure within the battery. Similarly, over-current can result in lithium ions not being embedded properly, leading to the formation of lithium metal on the electrode’s surface, which may cause a short circuit and an explosion. Ultrasonic welding and spot welding can also trigger explosions by melting the internal diaphragm of the battery or causing severe internal short circuits. Additionally, over-discharge can lead to the dissolution of negative electrode copper, potentially causing a short circuit and an explosion. Moreover, vibration and falls can misplace internal components of the battery, resulting in direct and severe shorts that can lead to explosions. It is essential to understand and mitigate these risks to prevent lithium battery explosions.
What are some situations of explosion in the production and use of lithium batteries?
Some situations that can lead to explosions in the production and use of lithium batteries include overcharging, overcurrent, ultrasonic welding of the plastic shell, spot welding, over-discharge, and exposure to vibration and falls. Overcharging can cause violent reactions inside the battery, leading to an explosion due to increasing pressure. Overcurrent, although rare, can occur when the charging current is too large, causing a direct short circuit and explosion. During ultrasonic welding of the plastic shell, excessive ultrasonic energy can lead to the internal diaphragm melting and a direct short circuit between positive and negative electrodes. Spot welding with too much current can result in a serious internal short circuit and explosion. Over-discharge, especially in conditions of over-charging or over-discharging with high current rates, can cause the negative electrode to dissolve and deposit on the diaphragm, leading to a short circuit and explosion. Lastly, exposure to violent vibrations or falls can misplace the internal components of the battery, resulting in a severe short circuit and potential explosion. These situations highlight the importance of careful handling and monitoring of lithium batteries to prevent such incidents.
What occurs in a battery at rest after fast charging?
The study shows that rapid charging of a battery can lead to the deposition of lithium ions in the form of lithium metal on the graphite particles. This phenomenon occurs when the graphite is fully charged, causing it to expand slightly. By closely monitoring the local lithiation in the electrode, researchers can determine the current flow in the battery at the particle level. This insightful approach provides valuable insights into the behavior of a battery at rest after fast charging, revealing crucial details about lithium ion deposition and volume changes that occur within the electrode material.
How do large local currents inside batteries at rest after fast charging contribute to thermal runaway?
Large local currents inside batteries at rest after fast charging contribute to thermal runaway by influencing the internal currents at the particle level. Research has shown that these local currents can reach current densities as high as 25 milliamps per centimeter squared after just a 10-minute charging period. These internal currents represent a significant factor in the occurrence of thermal runaway, indicating that they play a crucial role in the overall process.
Why has it been unclear why some batteries go into thermal runaway even when an electric vehicle is parked?
The reason for the uncertainty surrounding why certain batteries experience thermal runaway when an electric vehicle is idle is primarily due to the lack of a dependable technique for measuring internal currents within a stationary battery. This absence of a reliable method hinders researchers and engineers from accurately pinpointing the precise causes behind these incidents of thermal runaway in parked electric vehicles.
How do scientists gain insight into why thermal runaway in batteries could cause overheating and fires?
Scientists gain insight into why thermal runaway in batteries could cause overheating and fires by leveraging a technique known as ‘operando X-ray microtomography.’ This technique allows scientists to measure changes in the state of charge at the particle level within a lithium-ion battery after it has been charged quickly. By using operando X-ray microtomography, researchers can observe and analyze the internal behavior of the battery during different charging scenarios, providing valuable data to understand the mechanisms that lead to thermal runaway. This method offers a detailed look into the processes occurring within the battery at a microscopic level, helping scientists identify potential triggers for overheating and fire risks associated with thermal runaway in lithium-ion batteries.
Can overcharge, over-current, ultrasonic welding, spot welding, over-discharge, vibration, falls, and other factors cause lithium battery explosions?
Various factors can indeed cause lithium battery explosions, including overcharge, over-current, ultrasonic welding, spot welding, over-discharge, vibration, falls, and other circumstances.
An overcharge can lead to an explosion when the charging voltage exceeds a certain threshold, causing the electrolyte within the battery to decompose and trigger violent reactions, ultimately leading to an increase in pressure and a potential explosion. Similarly, an over-current situation can result in an explosion if the charging current becomes too excessive, preventing lithium ions from being embedded properly and causing a direct short circuit.
Ultrasonic welding of plastic shells, especially if not performed correctly, can transmit excessive energy to the battery cell, leading to internal reactions and a possible explosion. Spot welding, if done with too much current, can also cause serious internal shorts that may result in an explosion.
Furthermore, over-discharging a battery can lead to explosions, particularly if certain conditions are met, such as the dissolution and deposition of copper on the diaphragm, leading to a short circuit. Vibrations and falls can also potentially cause explosions by dislodging internal components and causing severe short circuits.
It is crucial to understand that these factors can contribute to the unsafe operation of lithium batteries, potentially leading to explosions and other hazardous situations. Proper handling, monitoring, and control of these factors are essential to ensure the safe use of lithium batteries and prevent such incidents from occurring.
What are some situations of explosion in the production and use of lithium batteries?
Certainly! Lithium batteries can explode in various situations during their production and use. Some of these situations include overcharge explosions, over-current explosions, explosions during ultrasonic welding of the plastic shell, explosions during spot welding, over-discharge explosions, and explosions due to vibration or falls. These incidents can occur when factors such as excessive charging voltage, uncontrolled charging current, ultrasonic energy transfer during welding, large welding currents, over-discharging, and physical impacts like vibration or falls lead to internal short circuits within the battery, causing violent reactions, pressure build-up, and ultimately resulting in explosions. It is crucial to understand and address these potential scenarios to enhance the safety of lithium battery production and usage.
What are some factors that can lead to battery explosion?
Several factors can lead to a battery explosion. Large internal polarization within the battery can trigger a chain reaction where the pole absorbs water and reacts with the electrolyte, causing the battery to swell due to gas generation. The quality and performance of the electrolyte play a crucial role in preventing such incidents. Issues with the liquid injection process, such as injecting an incorrect amount or poor sealing performance during assembly, can also contribute to a battery explosion. Additionally, the thickness of electrode pieces can disrupt the battery assembly process and impact its overall stability. External factors like high ambient temperatures can further exacerbate the risk of explosion. Dust accumulation or debris on the pole piece may create micro-short circuits, increasing the likelihood of a battery explosion. Proper attention to these factors is essential to prevent dangerous incidents from occurring.
Can lithium batteries explode due to overcharge, overcurrent and other factors?
Yes, lithium batteries can potentially explode due to various factors such as overcharge, overcurrent, and short circuits caused by factors like ultrasonic welding and spot welding. Overcharging a lithium battery can lead to the precipitation of lithium metal on its electrodes, causing internal shorts and potentially leading to an explosion. Similarly, overcurrent situations generate excessive heat and pressure within the battery, which can also result in an explosion. Factors like ultrasonic welding, spot welding, vibration, falls, and other external stresses can potentially damage the battery’s structure, leading to internal shorts and hazardous situations that may trigger an explosion. Therefore, it is crucial to handle lithium batteries carefully and ensure that they are not subjected to conditions that may increase the risk of explosion.
What are some situations of explosion in the production and use of lithium batteries?
In the production and use of lithium batteries, explosions may occur in various situations. One common reason is overcharge explosion, which happens when the charging voltage exceeds a safe limit, leading to violent reactions and pressure build-up within the battery. Another potential cause is over-current explosion, which occurs when the charging current is too high, preventing proper embedding of lithium ions and resulting in a short circuit.