- Forklift Lithium Battery
-
48V
- 48V 210Ah
- 48V 300Ah
- 48V 420Ah (949 x 349 x 569 mm)
- 48V 420Ah (950 x 421 x 450 mm)
- 48V 456Ah
- 48V 460Ah (830 x 630 x 590 mm)
- 48V 460Ah (950 x 421 x 450 mm)
- 48V 460Ah (800 x 630 x 600 mm)
- 48V 460Ah (820 x 660 x 470 mm)
- 48V 500Ah
- 48V 560Ah (810 x 630 x 600 mm)
- 48V 560Ah (950 x 592 x 450 mm)
- 48V 600Ah
- 48V 630Ah
-
48V
- Lithium Golf Cart Battery
- 12V Lithium Battery
12V 150Ah Lithium RV Battery
Bluetooth App | BCI Group 31
LiFePO4 Lithium
Discharge Temperature -20°C ~ 65°C
Fast Charger 14.6V 50A
Solar MPPT Charging - 24V Lithium Battery
- 36V Lithium Battery
- 48V Lithium Battery
-
48V LiFePO4 Battery
- 48V 50Ah
- 48V 50Ah (for Golf Carts)
- 48V 60Ah (8D)
- 48V 100Ah (8D)
- 48V 100Ah
- 48V 100Ah (Discharge 100A for Golf Carts)
- 48V 100Ah (Discharge 150A for Golf Carts)
- 48V 100Ah (Discharge 200A for Golf Carts)
- 48V 150Ah (for Golf Carts)
- 48V 160Ah (Discharge 100A for Golf Carts)
- 48V 160Ah (Discharge 160A for Golf Carts)
-
48V LiFePO4 Battery
- 60V Lithium Battery
-
60V LiFePO4 Battery
- 60V 20Ah
- 60V 30Ah
- 60V 50Ah
- 60V 50Ah (Small Size / Side Terminal)
- 60V 100Ah (for Electric Motocycle, Electric Scooter, LSV, AGV)
- 60V 100Ah (for Forklift, AGV, Electric Scooter, Sweeper)
- 60V 150Ah (E-Motocycle / E-Scooter / E-Tricycle / Tour LSV)
- 60V 200Ah (for Forklift, AGV, Electric Scooter, Sweeper)
-
60V LiFePO4 Battery
- 72V~96V Lithium Battery
- Rack-mounted Lithium Battery
- E-Bike Battery
- All-in-One Home-ESS
- Wall-mount Battery ESS
-
Home-ESS Lithium Battery PowerWall
- 24V 100Ah 2.4kWh PW24100-S PowerWall
- 48V 50Ah 2.4kWh PW4850-S PowerWall
- 48V 50Ah 2.56kWh PW5150-S PowerWall
- 48V 100Ah 5.12kWh PW51100-F PowerWall (IP65)
- 48V 100Ah 5.12kWh PW51100-S PowerWall
- 48V 100Ah 5.12kWh PW51100-H PowerWall
- 48V 200Ah 10kWh PW51200-H PowerWall
- 48V 300Ah 15kWh PW51300-H PowerWall
PowerWall 51.2V 100Ah LiFePO4 Lithium Battery
Highly popular in Asia and Eastern Europe.
CE Certification | Home-ESS -
Home-ESS Lithium Battery PowerWall
- Portable Power Stations
How Did the Battery Evolve into Lithium-Ion Technology?

The evolution of batteries into lithium-ion technology represents a significant leap in energy storage solutions. Beginning with early experiments in the 19th century, the development of lithium-ion batteries has been marked by innovations in materials and design, leading to their widespread adoption in consumer electronics, electric vehicles, and renewable energy systems. Understanding this evolution highlights the importance of lithium-ion technology in modern applications.
What Were the Early Developments in Battery Technology?
The journey towards lithium-ion technology began with the discovery of electricity and early battery designs. The first true battery, the voltaic pile, was created by Alessandro Volta in 1800. This laid the groundwork for subsequent developments, including lead-acid batteries in 1859 and nickel-cadmium batteries in the late 19th century.
Chart: Timeline of Early Battery Developments
| Year | Development |
|---|---|
| 1800 | Alessandro Volta invents the voltaic pile |
| 1859 | Invention of the lead-acid battery |
| 1899 | Introduction of nickel-cadmium batteries |
How Did Researchers Begin Exploring Lithium as a Battery Material?
Interest in lithium as a battery material emerged in the mid-20th century due to its high electrochemical potential and low weight. In 1976, M. Stanley Whittingham proposed using lithium titanium disulfide as a cathode material, although early designs faced safety issues due to lithium’s reactivity.
Chart: Key Milestones in Lithium Research
| Year | Researcher | Contribution |
|---|---|---|
| 1976 | M. Stanley Whittingham | Proposed lithium titanium disulfide as a cathode |
| 1980 | John B. Goodenough | Discovered lithium cobalt oxide for higher voltage |
| 1985 | Akira Yoshino | Developed the first commercial lithium-ion battery |
What Innovations Led to the First Commercial Lithium-Ion Battery?
The breakthrough for lithium-ion technology came through several key innovations:
- Lithium Cobalt Oxide: John Goodenough’s discovery of this material allowed for higher energy density.
- Graphite Anodes: Akira Yoshino replaced lithium metal with graphite, enhancing safety and performance.
- Commercialization: In 1991, Sony introduced the first commercial lithium-ion battery, marking a pivotal moment in battery technology.
Chart: Evolution of Lithium-Ion Battery Components
| Component | Original Material | Improved Material |
|---|---|---|
| Cathode | Lithium titanium disulfide | Lithium cobalt oxide |
| Anode | Lithium metal | Graphite |
Why Are Lithium-Ion Batteries Preferred Over Traditional Batteries?
Lithium-ion batteries offer several advantages over traditional lead-acid and nickel-cadmium batteries:
- Higher Energy Density: They can store more energy per unit weight, making them lighter and more efficient.
- Longer Lifespan: With proper management, they can last up to ten years or more.
- Low Self-Discharge Rate: They retain charge longer when not in use.
Chart: Comparison of Battery Types
| Battery Type | Energy Density (Wh/kg) | Lifespan (Years) | Self-Discharge Rate (%) |
|---|---|---|---|
| Lead-Acid | 30-50 | 3-5 | 10-15 |
| Nickel-Cadmium | 40-60 | 2-5 | 10-20 |
| Lithium-Ion | 150-250 | 8-10 | <5 |
How Have Safety Features Improved in Lithium-Ion Batteries?
Safety improvements have been integral to the evolution of lithium-ion technology. Early models faced risks such as thermal runaway and fires due to dendrite formation and overheating. Modern batteries incorporate:
- Battery Management Systems (BMS): These systems monitor temperature, voltage, and current to prevent unsafe conditions.
- Thermal Protection: Many designs include features that dissipate heat effectively.
What Future Innovations Are Expected in Lithium-Ion Technology?
The future of lithium-ion technology looks promising with ongoing research focused on:
- Solid-State Batteries: These replace liquid electrolytes with solid materials, enhancing safety and energy density.
- Recycling Technologies: Improved methods for recycling lithium-ion batteries are being developed to reduce environmental impact.
- Alternative Materials: Researchers are exploring new materials that could lower costs and improve performance.
Chart: Future Trends in Battery Technology
| Trend | Description |
|---|---|
| Solid-State Batteries | Higher safety and energy density |
| Enhanced Recycling | Reducing waste and recovering valuable materials |
| Alternative Materials | Exploring cheaper options like sodium-ion |
Industrial News
Recent advancements in battery technology highlight a growing focus on sustainability and efficiency. Companies are investing heavily in research to develop solid-state batteries that promise higher safety standards and energy density. Additionally, there is an increasing emphasis on recycling technologies aimed at minimizing waste from used batteries. This shift is expected to drive further innovations across various industries reliant on energy storage solutions.
Redway Power Expert Views
“Understanding the evolution of battery technology is crucial for grasping its current applications and future potential. As we continue to innovate within this field, we can expect even greater advancements that will enhance performance while addressing environmental concerns,” states an expert from Redway Power.

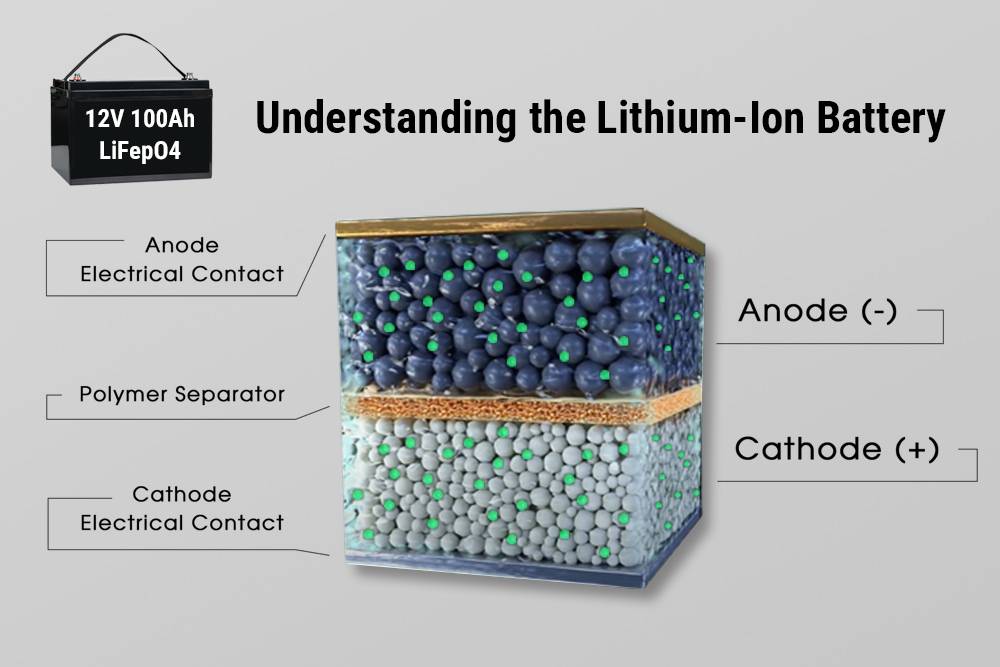

FAQ Section
Q: What is the main advantage of lithium-ion batteries?
A: The main advantage is their high energy density, allowing for longer usage times and lighter weights compared to traditional batteries.Q: Are lithium-ion batteries safe?
A: Yes, modern lithium-ion batteries have numerous safety features, including thermal protection and battery management systems that reduce risks.Q: How long do lithium-ion batteries last?
A: With proper care, they can last between 8 to 10 years, significantly longer than traditional lead-acid batteries.
Why Lithium Battery Prices Vary
What Influences Lithium Battery Costs
How Environmentally Friendly are Lithium Batteries
1992’s Consumer Electronics Battery Breakthrough
When Did Sony Begin Lithium-Ion Battery R&D
Sony began their lithium-ion battery research and development (R&D) in the early 1990s. They recognized the potential of this technology and successfully commercialized the first lithium-ion battery in 1991. Sony’s early investment and innovation in lithium-ion batteries played a crucial role in shaping the future of portable electronics and revolutionizing battery technology.
How Graphite Intercalation Advanced Battery Tech
Graphite intercalation is a process that involves inserting lithium ions between the layers of graphite in a battery electrode. This advancement in battery technology enabled higher energy density and improved performance in lithium-ion batteries. By allowing for the reversible intercalation of lithium ions, graphite electrodes played a crucial role in the widespread adoption of lithium-ion batteries in consumer electronics, electric vehicles, and renewable energy storage.
What Safety Risks Did Early Lithium Pose
Early lithium batteries posed safety risks due to the potential for thermal runaway, overheating, and explosions. The use of highly reactive materials, like lithium metal, increased the risk of short circuits and instability. However, advancements in battery technology have addressed these risks through the use of more stable materials and improved safety features, making modern lithium batteries much safer for use.
Who Created the First Lithium Battery
The first lithium battery was created through the collaborative efforts of M. Stanley Whittingham, John Goodenough, and Akira Yoshino. Whittingham’s discovery of intercalation electrodes, Goodenough’s development of the cathode material, and Yoshino’s successful commercialization of the lithium-ion battery were instrumental in the creation of the first practical lithium battery.





















