- Rack-mounted Lithium Battery
- Golf Cart Lithium Battery
-
Golf Cart Lithium Battery
- 36V 50Ah (for Golf Carts)
- 36V 80Ah (for Golf Carts)
- 36V 100Ah (for Golf Carts)
- 48V 50Ah (for Golf Carts)
- 48V 100Ah (Discharge 100A for Golf Carts)
- 48V 100Ah (Discharge 150A for Golf Carts)
- 48V 100Ah (Discharge 200A for Golf Carts)
- 48V 120Ah (for Golf Carts)
- 48V 150Ah (for Golf Carts)
- 48V 160Ah (Discharge 100A for Golf Carts)
- 48V 160Ah (Discharge 160A for Golf Carts)
-
Golf Cart Lithium Battery
- Forklift Lithium Battery
- 12V Lithium Battery
- 24V Lithium Battery
- 36V Lithium Battery
- 48V Lithium Battery
-
48V LiFePO4 Battery
- 48V 50Ah
- 48V 50Ah (for Golf Carts)
- 48V 60Ah (8D)
- 48V 100Ah (8D)
- 48V 100Ah
- 48V 100Ah (Discharge 100A for Golf Carts)
- 48V 100Ah (Discharge 150A for Golf Carts)
- 48V 100Ah (Discharge 200A for Golf Carts)
- 48V 150Ah (for Golf Carts)
- 48V 160Ah (Discharge 100A for Golf Carts)
- 48V 160Ah (Discharge 160A for Golf Carts)
-
48V LiFePO4 Battery
- 60V Lithium Battery
-
60V LiFePO4 Battery
- 60V 20Ah
- 60V 30Ah
- 60V 50Ah
- 60V 50Ah (Small Size / Side Terminal)
- 60V 100Ah (for Electric Motocycle, Electric Scooter, LSV, AGV)
- 60V 100Ah (for Forklift, AGV, Electric Scooter, Sweeper)
- 60V 150Ah (E-Motocycle / E-Scooter / E-Tricycle / Tour LSV)
- 60V 200Ah (for Forklift, AGV, Electric Scooter, Sweeper)
-
60V LiFePO4 Battery
- 72V~96V Lithium Battery
- E-Bike Battery
- All-in-One Home-ESS
- Wall-mount Battery ESS
-
Home-ESS Lithium Battery PowerWall
- 24V 100Ah 2.4kWh PW24100-S PowerWall
- 48V 50Ah 2.4kWh PW4850-S PowerWall
- 48V 50Ah 2.56kWh PW5150-S PowerWall
- 48V 100Ah 5.12kWh PW51100-F PowerWall (IP65)
- 48V 100Ah 5.12kWh PW51100-S PowerWall
- 48V 100Ah 5.12kWh PW51100-H PowerWall
- 48V 200Ah 10kWh PW51200-H PowerWall
- 48V 300Ah 15kWh PW51300-H PowerWall
PowerWall 51.2V 100Ah LiFePO4 Lithium Battery
Highly popular in Asia and Eastern Europe.
CE Certification | Home-ESS -
Home-ESS Lithium Battery PowerWall
- Portable Power Stations
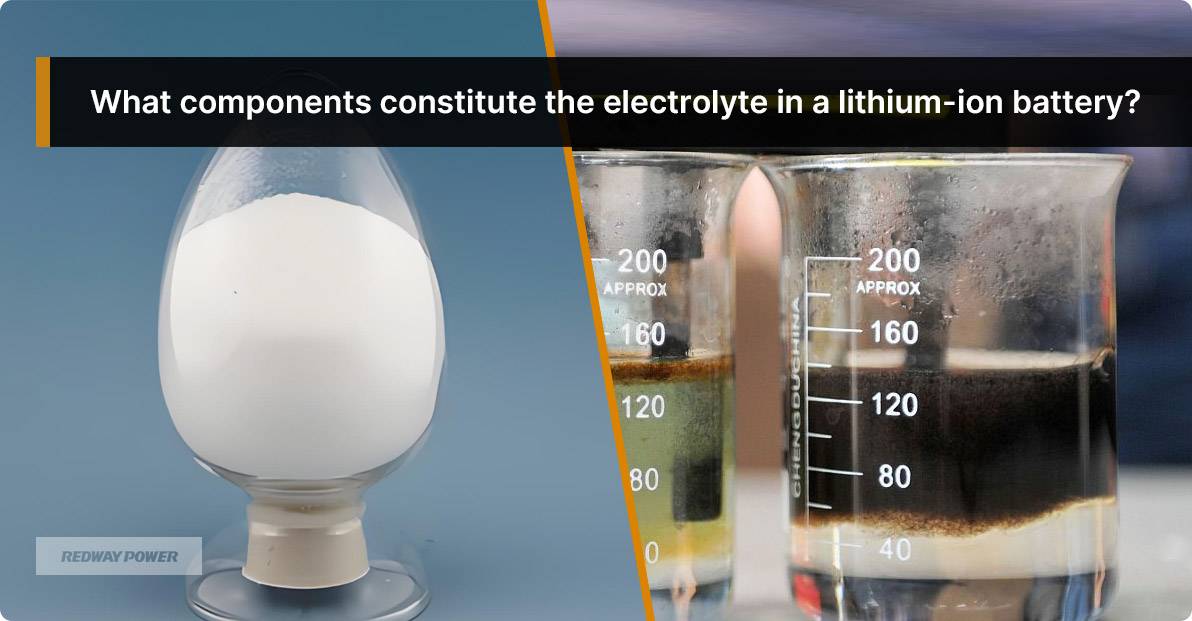
How Electrolytes Influence Battery Technology: From Lithium-Ion to Lead Acid

Electrolytes play a crucial role in battery technology, facilitating the movement of ions between electrodes to generate electrical energy. Understanding how electrolytes function across different battery types, such as lithium-ion and lead-acid, is essential for improving performance and safety.
What is the role of electrolytes in battery technology?
Electrolytes are substances that allow ions to flow between the positive and negative electrodes within a battery, enabling electrochemical reactions that produce electricity. Without an effective electrolyte, a battery cannot operate efficiently, as it serves as a medium for ion transport during charge and discharge cycles.Key Functions of Electrolytes:
- Ion Transport: Facilitates movement of ions between electrodes.
- Current Flow: Enables electrical current to pass through the battery.
- Chemical Reactions: Participates in reactions that generate electrical energy.
| Function | Description |
|---|---|
| Ion Transport | Moves cations and anions between electrodes |
| Current Flow | Allows electric current to flow through the circuit |
| Chemical Reactions | Engages in reactions that produce electricity |
How do electrolytes function in lithium-ion and lead-acid batteries?
In lithium-ion batteries, the electrolyte typically consists of lithium salts dissolved in organic solvents, allowing lithium ions to move between electrodes during charging and discharging. In contrast, lead-acid batteries use a mixture of sulfuric acid and water as the electrolyte, facilitating lead ion movement.Lithium-Ion Battery Functionality:
- Lithium ions migrate from the anode to cathode during discharge.
- The organic solvent provides high ionic conductivity but poses flammability risks.
Lead-Acid Battery Functionality:
- Lead ions move between lead dioxide (cathode) and sponge lead (anode).
- Sulfuric acid acts as an efficient medium for ion transport.
| Battery Type | Electrolyte Composition | Ion Movement |
|---|---|---|
| Lithium-Ion | Lithium salt in organic solvent | Lithium ions between electrodes |
| Lead-Acid | Sulfuric acid and water | Lead ions between lead plates |
What types of electrolytes are commonly used in batteries?
There are several types of electrolytes utilized across various battery technologies:
- Liquid Electrolytes: Commonly found in lead-acid and alkaline batteries; they consist of aqueous or organic solutions containing dissolved salts.
- Solid Electrolytes: Used in solid-state batteries; these materials provide enhanced safety and stability.
- Gel Electrolytes: A thickened form used primarily in sealed lead-acid batteries for leak resistance.
Comparison of Electrolyte Types:
| Type | Description | Applications |
|---|---|---|
| Liquid | Aqueous or organic solutions with dissolved salts | Lead-acid, alkaline batteries |
| Solid | Inorganic materials allowing ion passage | Solid-state batteries |
| Gel | Thickened liquid providing leak resistance | Sealed lead-acid batteries |
Why is electrolyte composition significant for battery efficiency?
The composition of an electrolyte directly impacts a battery’s efficiency, affecting factors such as ionic conductivity, thermal stability, and overall performance.Key Components Influencing Efficiency:
- Solvents: Affect viscosity and ionic conductivity.
- Salts: Determine the electrochemical stability and voltage range.
- Additives: Enhance performance characteristics like cycle life and safety.
| Component | Impact on Efficiency |
|---|---|
| Solvents | Influence viscosity and ionic conductivity |
| Salts | Determine voltage stability |
| Additives | Improve cycle life and safety |
How do electrolyte properties affect overall battery performance?
Electrolyte properties such as conductivity, stability, viscosity, and temperature tolerance play crucial roles in determining how well a battery performs under various conditions.Essential Properties:
- Conductivity: Higher conductivity leads to lower internal resistance.
- Stability: Chemically stable electrolytes prevent degradation over time.
- Viscosity: Affects the speed at which ions can move through the electrolyte.
What are the safety considerations regarding battery electrolytes?
Safety is paramount when dealing with battery electrolytes due to potential hazards associated with their chemical compositions:
- Flammability: Organic solvents used in lithium-ion batteries can be highly flammable.
- Corrosiveness: Sulfuric acid in lead-acid batteries poses risks to skin and eyes.
- Toxicity: Some components may release harmful gases during malfunction or leakage.
Safety Measures:
- Use protective gear when handling batteries.
- Store batteries properly to avoid leaks or spills.
- Ensure proper ventilation when charging or discharging batteries.
What recent advancements are being made in electrolyte technology?
Recent innovations focus on developing safer and more efficient electrolyte materials to enhance battery performance:
- Solid-State Electrolytes: These reduce flammability risks associated with liquid electrolytes.
- Advanced Organic Solvents: Research is ongoing into non-flammable organic solvents for lithium-ion applications.
- Hybrid Electrolyte Systems: Combining liquid and solid-state technologies aims to optimize performance while maintaining safety.
What expert insights can enhance understanding of battery electrolytes?
“Understanding the diverse roles that electrolytes play across various battery technologies is essential for advancing energy storage solutions,” notes an expert from Redway Power Insights. “As we innovate new materials and compositions, we can improve both performance metrics and safety standards.”Redway Power Insights:
“Future developments will likely focus on integrating solid-state technologies with existing systems to create safer, more efficient energy storage solutions capable of meeting growing global demands.”
Industrial News
Recent reports indicate a surge in research focused on solid-state batteries due to their enhanced safety profiles compared to traditional lithium-ion technologies. Companies are investing heavily in developing solid-state solutions that promise higher energy densities while minimizing risks associated with liquid electrolytes. Additionally, regulatory bodies are emphasizing sustainable practices within the industry, pushing for advancements that reduce environmental impacts during production and recycling processes.
FAQ Section
Q1: What is the primary function of an electrolyte?
A1: The primary function of an electrolyte is to facilitate ion transport between electrodes during a battery’s charge and discharge cycles.Q2: Why are solid-state electrolytes considered safer?
A2: Solid-state electrolytes eliminate flammability risks associated with liquid electrolytes while providing enhanced stability under varying temperatures.Q3: How does electrolyte composition affect a battery’s lifespan?
A3: The right combination of solvents, salts, and additives can significantly enhance a battery’s lifespan by improving its electrochemical stability and reducing degradation rates.