- Rack-mounted Lithium Battery
- Golf Cart Lithium Battery
-
Golf Cart Lithium Battery
- 36V 50Ah (for Golf Carts)
- 36V 80Ah (for Golf Carts)
- 36V 100Ah (for Golf Carts)
- 48V 50Ah (for Golf Carts)
- 48V 100Ah (Discharge 100A for Golf Carts)
- 48V 100Ah (Discharge 150A for Golf Carts)
- 48V 100Ah (Discharge 200A for Golf Carts)
- 48V 120Ah (for Golf Carts)
- 48V 150Ah (for Golf Carts)
- 48V 160Ah (Discharge 100A for Golf Carts)
- 48V 160Ah (Discharge 160A for Golf Carts)
-
Golf Cart Lithium Battery
- Forklift Lithium Battery
- 12V Lithium Battery
- 24V Lithium Battery
- 36V Lithium Battery
- 48V Lithium Battery
-
48V LiFePO4 Battery
- 48V 50Ah
- 48V 50Ah (for Golf Carts)
- 48V 60Ah (8D)
- 48V 100Ah (8D)
- 48V 100Ah
- 48V 100Ah (Discharge 100A for Golf Carts)
- 48V 100Ah (Discharge 150A for Golf Carts)
- 48V 100Ah (Discharge 200A for Golf Carts)
- 48V 150Ah (for Golf Carts)
- 48V 160Ah (Discharge 100A for Golf Carts)
- 48V 160Ah (Discharge 160A for Golf Carts)
-
48V LiFePO4 Battery
- 60V Lithium Battery
-
60V LiFePO4 Battery
- 60V 20Ah
- 60V 30Ah
- 60V 50Ah
- 60V 50Ah (Small Size / Side Terminal)
- 60V 100Ah (for Electric Motocycle, Electric Scooter, LSV, AGV)
- 60V 100Ah (for Forklift, AGV, Electric Scooter, Sweeper)
- 60V 150Ah (E-Motocycle / E-Scooter / E-Tricycle / Tour LSV)
- 60V 200Ah (for Forklift, AGV, Electric Scooter, Sweeper)
-
60V LiFePO4 Battery
- 72V~96V Lithium Battery
- E-Bike Battery
- All-in-One Home-ESS
- Wall-mount Battery ESS
-
Home-ESS Lithium Battery PowerWall
- 24V 100Ah 2.4kWh PW24100-S PowerWall
- 48V 50Ah 2.4kWh PW4850-S PowerWall
- 48V 50Ah 2.56kWh PW5150-S PowerWall
- 48V 100Ah 5.12kWh PW51100-F PowerWall (IP65)
- 48V 100Ah 5.12kWh PW51100-S PowerWall
- 48V 100Ah 5.12kWh PW51100-H PowerWall
- 48V 200Ah 10kWh PW51200-H PowerWall
- 48V 300Ah 15kWh PW51300-H PowerWall
PowerWall 51.2V 100Ah LiFePO4 Lithium Battery
Highly popular in Asia and Eastern Europe.
CE Certification | Home-ESS -
Home-ESS Lithium Battery PowerWall
- Portable Power Stations
What are the 3 main components of battery cells?

Battery cells consist of three main components: an anode, a cathode, and an electrolyte. The anode releases electrons, the cathode undergoes reduction, and the electrolyte allows ion flow. Together, these components work to produce electricity. Contact Redway Experts to learn more.
What Components Make a Battery?
Anode: Definition and Function
Cathode: Definition and Function
Electrolyte: Definition and Function
How Each Component Works Together to Produce Electricity
Advancements in Battery Cell Technology
Here is the article organized by the given headings and following the specified steps:
How does the separator in a battery system allow ions to pass through while isolating the electrodes?
The separator in a battery allows ions to pass through its porous structure while electrically insulating the positive and negative electrodes from each other, preventing a short circuit.
Batteries work by allowing ions to flow between electrodes during charging and discharging. The separator plays an important role by letting only ions pass through its thin porous material, usually made from plastic or ceramics. This allows ion exchange while preventing the electrodes from touching. If the electrodes touched, a short circuit would occur and the battery would no longer function. Different separator materials are used depending on the type of battery. For example, lithium-ion batteries often use polypropylene or polyethylene separators only a few microns thick. Their precise porous structure facilitates rapid ion transport while maintaining electrical isolation of the electrodes. This separator design is key to lithium-ion batteries’ widespread use in devices like phones and laptops.
How does the direction of current flow change between charging and discharging a battery?
A battery’s current flow reverses direction between charging and discharging – into the positive terminal and out the negative during charging, and out the positive and into the negative when powering a device on discharge.
Batteries provide power by allowing current to flow one way when charging and the opposite way when discharging. Here are the key points:
1. CHARGING: Current flows INTO the positive terminal and OUT of the negative terminal. This pumps ions into the electrodes to store energy.
2. DISCHARGING: The current REVERSES direction, flowing OUT of the positive terminal and INTO the negative terminal. This supplies power to a device from the stored energy.
3. This bi-directional current allows batteries to both receive and deliver electrical energy on demand. Without the reversal, batteries could only charge or discharge, not both.
4. Different types of batteries, like lead-acid in cars or lithium-ion in phones, all follow this same charging and discharging current direction pattern. It is fundamental to how batteries store and release power.
How do lithium-metal batteries differ from lithium-ion batteries in terms of electrode composition?
Lithium-ion batteries use carbon anodes whereas lithium-metal batteries use metallic lithium foil anodes, but both battery types commonly have lithium metal oxide cathodes.
Both lithium-ion and lithium-metal batteries are lithium-based battery types, but they differ in the composition of one key electrode:
1. ANODE: Lithium-ion batteries use a carbon-based material like graphite for the anode. Lithium-metal batteries instead employ metallic lithium foil.
2. CATHODE: The cathode is typically a lithium metal oxide compound for both battery types.
3. This difference arises from lithium metal’s reactivity – it forms dendrites on charge that can cause shorting. The graphite anode prevents this in lithium-ion batteries.
4. As a result, lithium-ion batteries have greater safety and cycle life, making them prevalent in consumer devices. Research continues on developing safe lithium-metal batteries for electric vehicles.
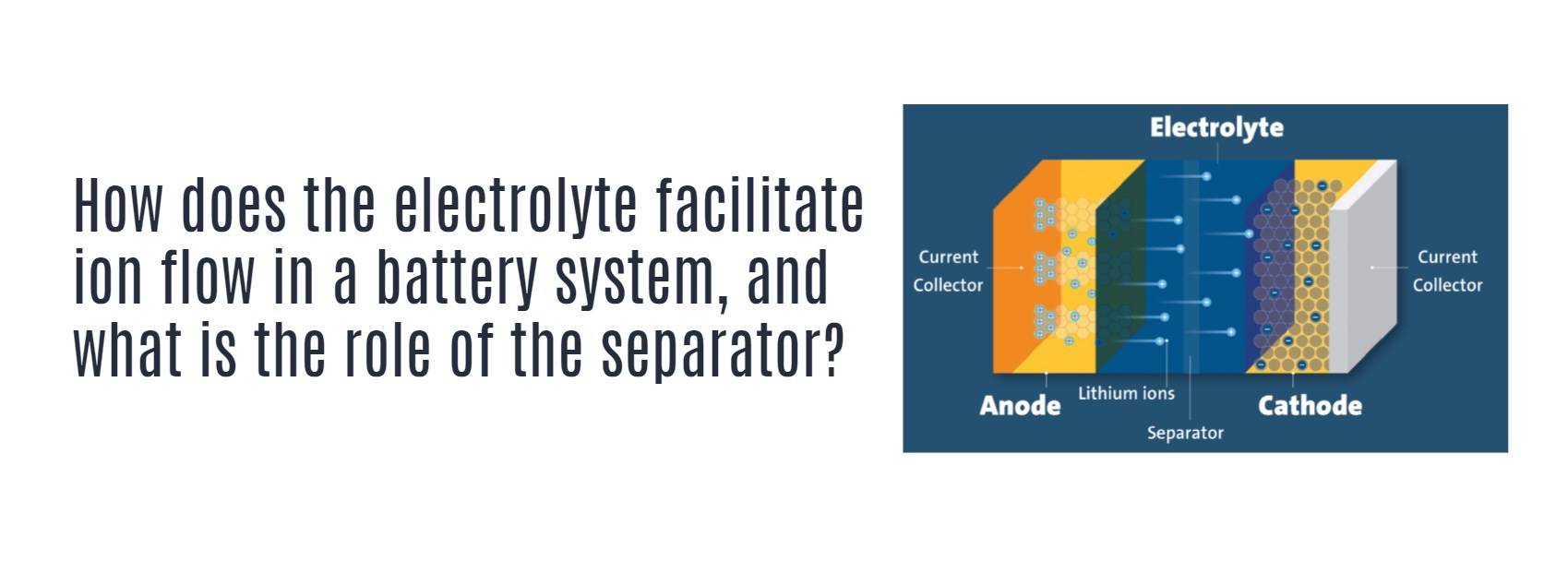
How does the electrolyte facilitate ion flow in a battery system, and what is the role of the separator?
An electrolyte facilitates ion flow between electrodes in a battery, while the separator permits ion passage through its porous structure to complete the circuit but blocks electrode contact to prevent shorting.
Batteries work through the movement of ions between electrodes. Here are the roles of two important components:
1. ELECTROLYTE: This liquid or gel contains ions that FLOW between the cathode and anode during charging and discharging. Its ions ferry the battery’s charge.
2. SEPARATOR: Sitting between electrodes, its porous nature ALLOWS ions to pass through while its insulating properties PREVENT the electrodes from PHYSICALLY TOUCHING. If they touched, a short would disable the battery.
3. Together these enable ion exchange while keeping electrodes isolated. Different types are used – lithium-ion often employs a polymer gel electrolyte and microporous plastic or ceramic separator.
4. Proper electrolyte/separator selection based on battery chemistry is vital for performance, safety and cycle life. Advanced materials continue improving these components.
FAQS
Why EMS is Essential in BESS
An Energy Management System (EMS) is crucial in a Battery Energy Storage System (BESS). It consists of sensors, meters, and controllers that track energy metrics in real-time. The EMS enables seamless communication between its digital directives and the BESS’s physical operations, ensuring precise energy management. This integration optimizes the performance of the BESS, allowing for cost-effective and efficient operation in coordination with other generation sources.














